APPLICATION NOTE: AN-006
Importance of Particle Size and Shape in Dissolution of Multi-Component Drug Delivery Systems
Purpose
The particle size and particle shape of multi-component controlled release drugs must be considered during their dissolution of as a function of time. Though the size and shape of the particles are well known when the capsules are filled, oftentimes, little is known about the changes in size, shape, and morphology during dissolution. Particles that start as spheres or granules will eventually take new shapes as they break down. Particle Shape analysis is used to show that the multiple components of the drug are similar in size but have distinct shapes as well as to demonstrate that particle shapes change as they dissolve.
Background
Particle size and shape are critical quality attributes of many pharmaceutical materials from API’s to excipients. The evolution of particle sizes and shapes as they dissolve over time is not completely understood for many released drugs as well as those in development. Oftentimes there are multiple active ingredients present. These multifunctional components and varying particle shapes present challenges to traditional dissolution testing devices. Additionally, the particle sizes and particle shapes of these components may have an impact on physicochemical properties, bioavailability and other pharmacokinetic phenomena. Applying many of the analytical tools available for size and shape analysis is on the rise and is being considered for many new drug formulations currently under development. New regulatory initiatives are forcing researchers to identify and employ new test methods that will provide a better understanding of material properties that can impact functionality.
Methods
The particle size of an encapsulated drug material as a controlled release delivery mechanism was determined using a Laser Diffraction instrument based on static light scattering technique. The particle shape was determined using the Pi Sentinel PRO which utilizes the dynamic image analysis technique. Dynamic image analysis provides additional utility in that it does not assume that all particles are spheres as do many commonly used particle size distribution analysis systems such as light scattering. In this study, we reported particle size using both techniques as well as various shape parameters that include Circularity, Smoothness, and Aspect ratio. In each case, the time release beads were suspended in an aqueous diluent where measurements were taken every 5 minutes, unless otherwise noted, for a period of one hour.
Results and Discussion
Figure 1 shows the change in surface Smoothness of a common OTC pain reliever as the particles dissolve over time. When suspended in the diluent, the drug granules break down and various components of the drug formulation emerge. In most cases, the data that scientists use to determine the particle size distribution of their materials assumes that all particles are spheres. The same OTC encapsulated pain reliever was dispersed in liquid and analyzed via dynamic image analysis to illustrate the true particle shape.
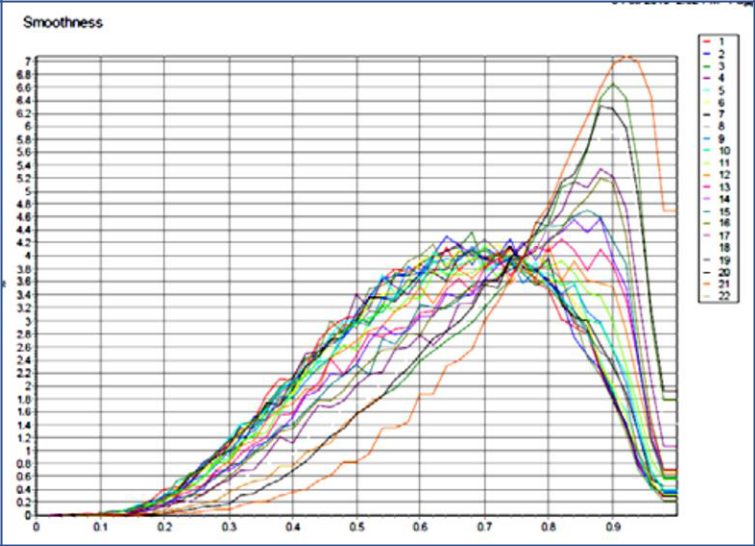
Figure 1: Results from Dynamic image analysis shows the particles are becoming smoother over time.
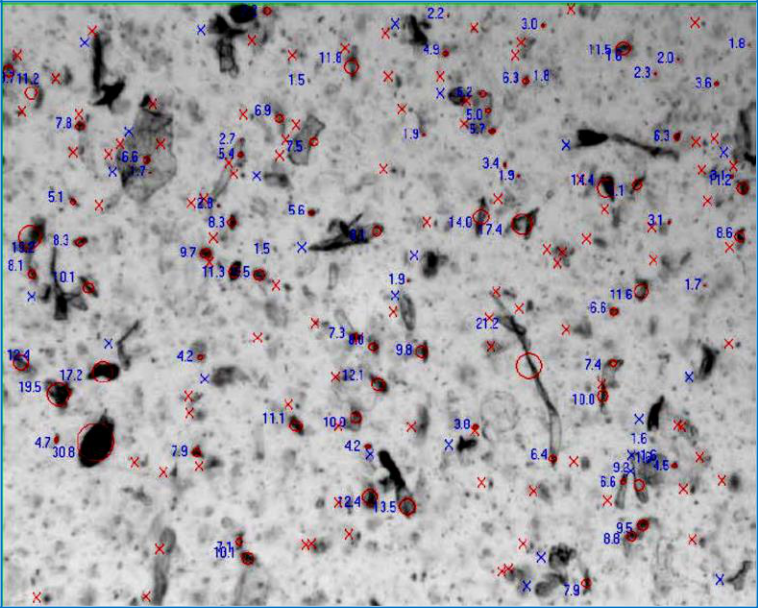
Figure 2: Dynamic image analysis data showing particle shape of a common OTC pain reliever.
Conclusion
Particles come in a variety of shapes and sizes. It is generally understood that modifications or changes in size and shape impact functionality. The data that was collected shows the effect of dissolution on the shape and size of the drug components. The data was found to be useful in determining the impact of dissolution on these two-key physical particle properties.

