USP <1788> Explained: How the Raptor 1788 Adds Dynamic Image Analysis to Subvisible Particle Testing
Subvisible particulate matter is under more scrutiny than ever in parenteral and ophthalmic drug products.
USP <1788> and <1788.3> now explicitly recommend flow imaging / dynamic image analysis (DIA) as a key orthogonal method alongside traditional light obscuration (LO) and membrane microscopy.
This guide explains what USP <1788> means, why it matters, and how the Vision Analytical Raptor 1788 helps pharmaceutical QC labs meet these expectations without replacing their existing LO system.
Contents
-
Why USP <1788> Matters
-
How USP <1788> Relates to USP <787>, <788>, and <789>
-
What USP <1788> Actually Says
-
USP <1788.3>: Flow Imaging / DIA for 2–100 µm
-
Limitations of Light Obscuration
-
Introducing the Raptor 1788
-
Key Use Cases
-
How to Implement USP <1788> in Your Lab
-
FAQ
-
Next Steps
1. Why USP <1788> Matters
Subvisible particles (typically 2–100 µm) are considered a critical quality attribute for parenteral and ophthalmic products. They help assess:
-
Foreign particulate contamination
-
Formulation stability (especially for biologics/proteins)
-
Container/closure integrity
-
Particles inherent to manufacturing processes
Traditional compendial methods (USP <787>, <788>, <789>) focus on numerical limits using LO and membrane microscopy. But these methods alone cannot fully characterize many particle types — especially protein aggregates, silicone oil droplets, fibers, and other irregular particles.
USP <1788> steps in to guide better testing practices and encourage orthogonal methods like DIA.
2. How USP <1788> Relates to USP <787>, <788>, and <789>
Think of the normative chapters as “what you must do”, and USP <1788> as “how to do it well.”
-
USP <787> – Therapeutic protein injections
-
USP <788> – Particulate matter in injections
-
USP <789> – Particulate matter in ophthalmic solutions
-
USP <1788> – Describes how to apply LO, microscopy, and imaging methods correctly
USP <1788> emphasizes:
-
Proper sample handling and mixing
-
Calibrations, system suitability, and method control
-
Strengths and limitations of each technology
-
The value of orthogonal methods like flow imaging/DIA
3. What USP <1788> Actually Says
USP <1788> covers three main analytical families:
-
Light obscuration (LO)
-
Membrane microscopy (MPC)
-
Flow imaging / dynamic image analysis (DIA) — detailed in USP <1788.3>
USP highlights several important themes:
1. Use multiple orthogonal techniques
LO is essential, but imaging provides particle identity, not just counts.
2. Method-dependent differences are normal
Different technologies “see” particles differently — and that’s expected.
3. Sample handling matters
Mixing, sample viscosity, container type, and environmental controls can dramatically change results.
4. USP <1788.3>: Flow Imaging / DIA in the 1–100 µm Range
USP <1788.3> provides technical guidance for imaging-based methods like the Raptor 1788.
Flow imaging / DIA typically covers:
-
2–100 µm for subvisible particles
-
Larger particles beyond 100 µm (instrument dependent)
-
Ability to discriminate particle types and morphologies
What imaging adds:
-
Shape descriptors (aspect ratio, circularity, elongation). The more shape descriptors available, the more capable software is to differentiate one particle population from another.
-
Opacity/brightness
-
Texture features
-
Thumbnail images of each particle
- Classification of particle types
This is crucial for biologics, where protein aggregates may be inherent but foreign contaminants are not.
5. Limitations of Light Obscuration Alone
LO remains the primary compendial method, but it cannot:
-
Identify particle type
-
Distinguish silicone oil droplets from solid particulates
-
Detect low-refractive-index (soft/transparent) particles well
-
Provide visual proof during investigations
-
Determine morphology
This is exactly why USP <1788> recommends orthogonal methods like DIA to complement LO.
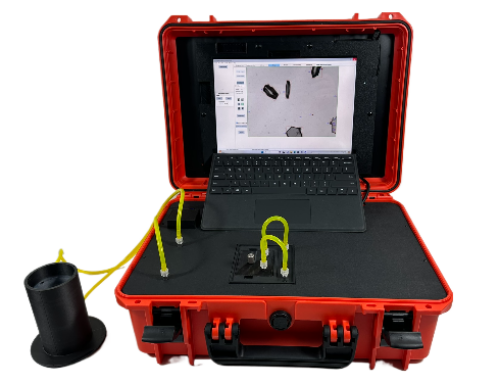
6. Introducing the Raptor 1788: DIA Optimized for USP <1788>
The Vision Analytical Raptor 1788 is a purpose-built dynamic image analysis module that enhances your existing LO workflow.
6.1 Dynamic Image Analysis for Subvisible Particles
Raptor 1788 captures detailed images of each particle and provides:
-
Multiple size metrics and shape descriptors of particles down to 1 micron due to high-resolution optical components.
-
Texture and brightness features
-
Image thumbnails for each detected particle
This enables classification such as:
-
Protein aggregates
-
Silicone oil droplets
-
Glass fragments
-
Fibers
-
Rubber or elastomer particles
-
Manufacturing/packaging contaminants
6.2 Retrofit, Don’t Replace
Raptor 1788 can operate stand-alone or can be integrated with your existing LO system:
-
No need to replace validated methods
-
Minimal workflow changes
-
Uses similar sample volumes
-
Allows LO to remain your primary compendial method
6.3 Built for USP <1788> and <1788.3>
-
Designed around the 1 – 100 µm range
-
Supports trending, risk assessments, and investigations
-
Provides the morphological detail USP recommends
-
Helps meet expectations for advanced biologics
7. Key Use Cases
7.1 Therapeutic Proteins & Biologics
-
Differentiate protein aggregates from contaminants
-
Trend aggregation under stress/storage
-
Support USP <1787>-recommended risk assessments
7.2 Cell & Gene Therapy / High-Value Low-Volume Products
-
Reduce sample volume needs
-
Extract maximum data per microliter
-
Analyze morphology-rich particle profiles
7.3 Pre-Filled Syringes & Silicone Oil
-
Identify and quantify silicone droplets
-
Distinguish droplets from harmful particulates
-
Study formulation–container interactions
- Run samples directly from your pre-filled syringe in order to eliminate external sources of contamination.
8. How to Implement USP <1788> with Raptor 1788
A practical rollout strategy:
Step 1 — Map your current testing
Identify where LO alone is insufficient (biologics, new containers, investigations).
Step 2 — Define where imaging adds value
Use Raptor 1788 for:
-
Investigations
-
Comparability studies
-
Stability studies beyond LO
-
Characterization of protein particles vs silicone
Step 3 — Develop a DIA method aligned with USP guidance
Include:
-
Mixing procedures
-
System suitability checks
-
Method verification
-
Data review criteria
Step 4 — Integrate Raptor 1788 into your LO workflow
LO stays your primary compendial test; DIA adds orthogonal confidence.
Step 5 — Use images for root-cause analysis
Build particle libraries and reduce deviations.
9. FAQ
Is USP <1788> mandatory?
No. It’s informational — but widely considered modern best practice.
Does Raptor 1788 replace my LO system?
No. It enhances LO by adding imaging and morphology.
What size range does Raptor 1788 cover?
Typically, 1–100 µm for subvisible particles (configuration dependent).
Can Raptor 1788 identify particle types?
Yes — via morphology, brightness, and image inspection.
Is this only for biologics?
No — it’s useful for any parenteral or ophthalmic product.
10. Next Steps: Evaluate Raptor 1788 for Your Lab
USP <1788> represents a shift from simply counting particles to actually understanding what they are.
With the Raptor 1788, you can:
-
Add dynamic image analysis without replacing your LO
-
Follow USP <1788> recommendations
-
Improve investigations and root-cause analysis
-
Future-proof your QC for advanced therapies
👉 Want help integrating USP <1788> into your workflow?
Reach out here: Contact Vision Analytical