DATA INTEGRITY
What is Data Integrity?
Data Integrity in Particle Size and Shape Analysis
Pharmaceutical companies require laboratory equipment that fully complies with data integrity standards set by regulatory agencies such as the U.S. Food and Drug Administration (FDA). Vision Analytical’s particle size and shape analyzers are designed to support these requirements, ensuring trustworthy results for quality control, research, and manufacturing environments.
FDA Definition of Data Integrity
According to the FDA’s guidance document, “Data Integrity and Compliance with CGMP … Guidance for Industry,” data integrity is defined as the completeness, consistency, and accuracy of data. Reliable pharmaceutical data must be:
-
Attributable – linked to the person generating the data
-
Legible – recorded clearly and permanently
-
Contemporaneously recorded – documented at the time of activity
-
Original or a true copy – maintaining authenticity
-
Accurate – correct, reliable, and free from manipulation
This set of principles is known by the acronym ALCOA, which is frequently cited in FDA guidance documents.
Why Data Integrity Matters in Pharmaceutical Particle Analysis
For pharmaceutical applications, particle size and shape measurements directly influence drug quality, safety, and efficacy. Non-compliant data handling can put both regulatory approval and patient safety at risk. That’s why data integrity practices—such as audit trails, electronic records, and secure data storage—are essential when using particle characterization equipment in regulated industries.
Vision Analytical’s Commitment to Compliance
Vision Analytical’s systems are developed to align with FDA data integrity expectations, 21 CFR Part 11 electronic records compliance, and CGMP guidelines. Features such as:
-
Comprehensive audit trails
-
Secure, traceable electronic records
-
True copies of original data
-
User accountability and access control
help ensure that your particle size and shape analysis results meet pharmaceutical industry standards for compliance, accuracy, and reliability.
How the Particle Insight complies …
IQ/OQ Qualification Program …
The Pi Sentinel PRO has a robust IQ/OQ Qualification Program which is to be performed by qualified Field Service Engineers (FSE) specially trained and certified by the factory in the execution of the qualification process.
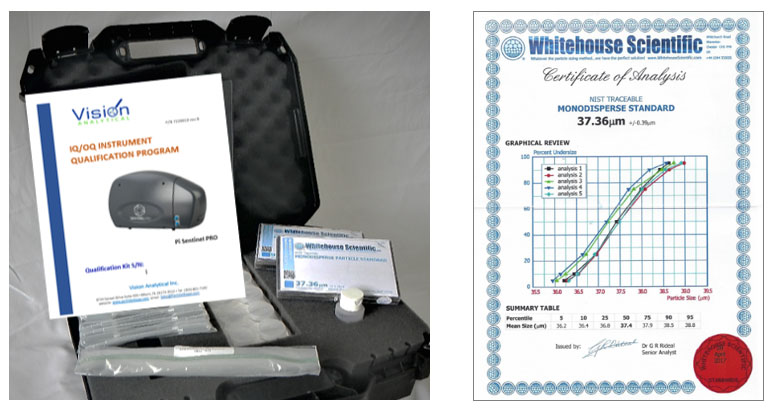
A Qualification Kit provides a set of documents and forms, including a DATA INTEGRITY form, supplies and NIST traceable Standards to complete the Qualification.
Data Integrity form indicates the Pi Sentinel PRO system was set up accordingly to the requirements set forth in Data Integrity section of the IQ/OQ Qualification Program.
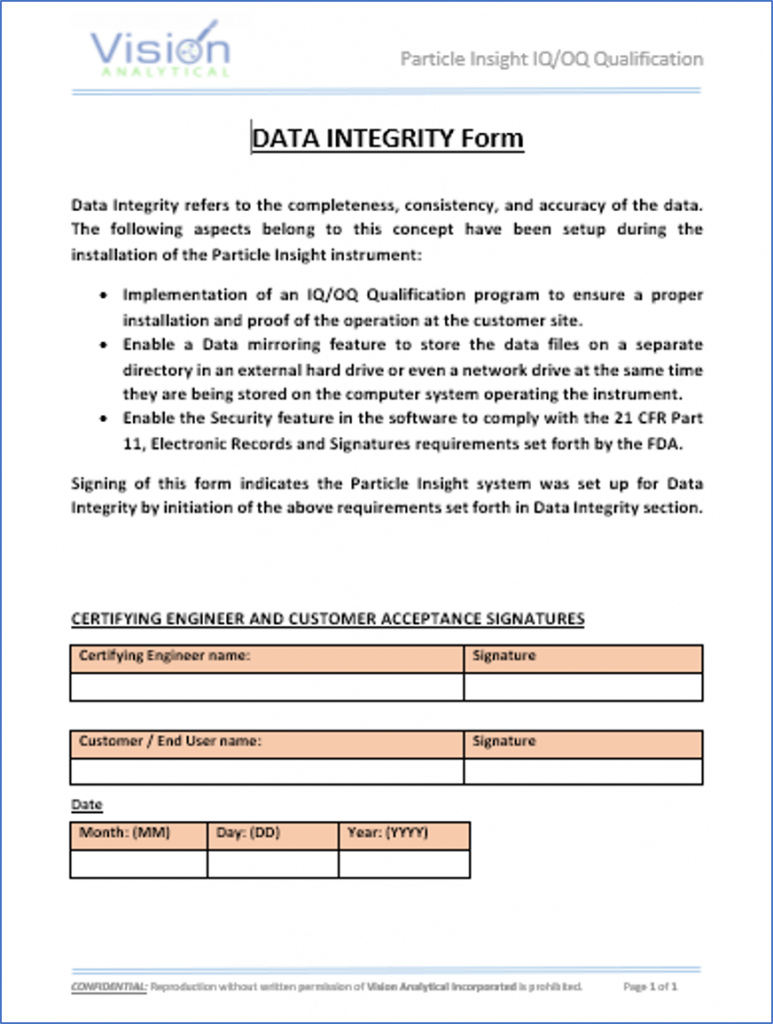
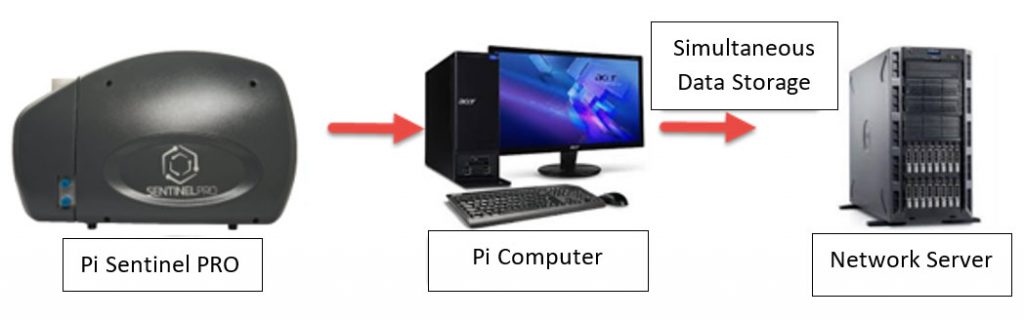
Data Mirroring …
Data Mirroring is a feature within the Pi Sentinel PRO software where the computer software will store the data files on a separate directory or hard drive or even a network drive at the same time it is being stored on the computer system operating the instrument.
For detailed information on how our products are compliant with Data Integrity document below,


