USP <1788.3>: A New Chapter in Particle Characterization
The United States Pharmacopeia (USP) has introduced <1788.3>, expanding guidance for subvisible particle analysis to include Dynamic Image Analysis (DIA) as a complementary, or orthogonal, method to traditional Light Obscuration (LO).
This update recognizes that size alone isn’t enough — particle shape, structure, and morphology are now critical for ensuring the quality and safety of injectable products.
With regulatory expectations shifting, manufacturers must enhance their existing LO workflows to provide visual confirmation of every particle — without disrupting validated systems.
The Problem: New USP <1788.3> Requirements for Injectable Quality
Pharmaceutical manufacturers face growing pressure to ensure injectable products are free from subvisible particulate matter — not just in size and count, but in shape and origin.
While Light Obscuration (USP <787>/<788>) remains the standard, it struggles to detect translucent or irregular particles such as protein aggregates or silicone oil droplets. These limitations prompted the USP to introduce <1788.3>, recommending Dynamic Image Analysis (DIA) as an orthogonal method that provides visual proof and morphology data for every particle detected.
However, most flow-imaging systems capable of meeting <1788.3> are expensive and slow and require full system replacement and revalidation. For QC labs under tight budgets and production timelines, this makes compliance challenging and costly.
The Smarter Path to USP <1788.3> Compliance
Enhance your existing Light Obscuration system — don’t replace it.
Instead of replacing your validated Light Obscuration system, enhance it. The Raptor 1788 easily integrates as a bolt-on Dynamic Image Analysis module, giving you the imaging power required by USP <1788.3>—without changing your workflow, revalidating equipment, or investing in a costly new system.
Meet the Raptor 1788
The Raptor 1788 delivers Dynamic Image Analysis (DIA) capability to your existing Light Obscuration system, transforming it into a powerful, dual-mode solution for USP <1788.3> compliance. This innovative add-on captures size, shape, concentration, classification, and visual images of every particle, giving you the clarity and confidence regulators now expect—without disrupting your validated workflow or increasing your sample processing time.

Key Benefits of the Raptor 1788
-
USP <1788.3> Compliance – Adds Dynamic Image Analysis to your existing Light Obscuration system for full regulatory coverage.
-
No Revalidation Required – Non-intrusive retrofit that keeps your validated LO workflow intact.
-
Visual Proof for Every Particle – Capture size, shape, and morphology images to identify and classify particulate matter.
-
Fast and Efficient – Analyze samples quickly with higher throughput than combined LO/Imaging systems.
-
Cost-Effective Upgrade – Achieve full compliance at a low cost compared to replacing your LO system.
-
Seamless Integration – Compact, bolt-on design compatible with most existing LO instruments.
- Flexibility – Can also be operated as a Stand-Alone system if needed. Can also analyze continuous samples directly from prefilled syringes or directly from IV bags.
USP <1788.3> Compliance – Adds Dynamic Image Analysis to your existing Light Obscuration system for full regulatory coverage.
No Revalidation Required – Non-intrusive retrofit that keeps your validated LO workflow intact.
Visual Proof for Every Particle – Capture size, shape, and morphology images to identify and classify particulate matter.
Fast and Efficient – Analyze samples quickly with higher throughput than combined LO/Imaging systems.
Cost-Effective Upgrade – Achieve full compliance at a low cost compared to replacing your LO system.
Seamless Integration – Compact, bolt-on design compatible with most existing LO instruments.
Compliance Made Simple
With the Raptor 1788, meeting the new USP <1788.3> requirements doesn’t have to mean replacing your existing system or starting over. Vision Analytical’s proven Dynamic Image Analysis technology empowers you to achieve compliance, gain visual insight, and improve product quality — all through a fast, affordable, and fully compatible add-on solution.
→ Request a demo or sample analysis today and see how easily you can enhance your current Light Obscuration workflow.

Coming Soon: Raptor 1788 Integration with Beckman HIAC 9703+
We’re taking compliance and performance even further. The Raptor 1788 will soon be available as a direct retrofit for the Beckman HIAC 9703+, the industry standard for USP <788> Light Obscuration testing. This seamless integration brings Dynamic Image Analysis directly into your trusted HIAC workflow — no revalidation, no replacement, just enhanced insight.
Check back soon to see this powerful combination in action.
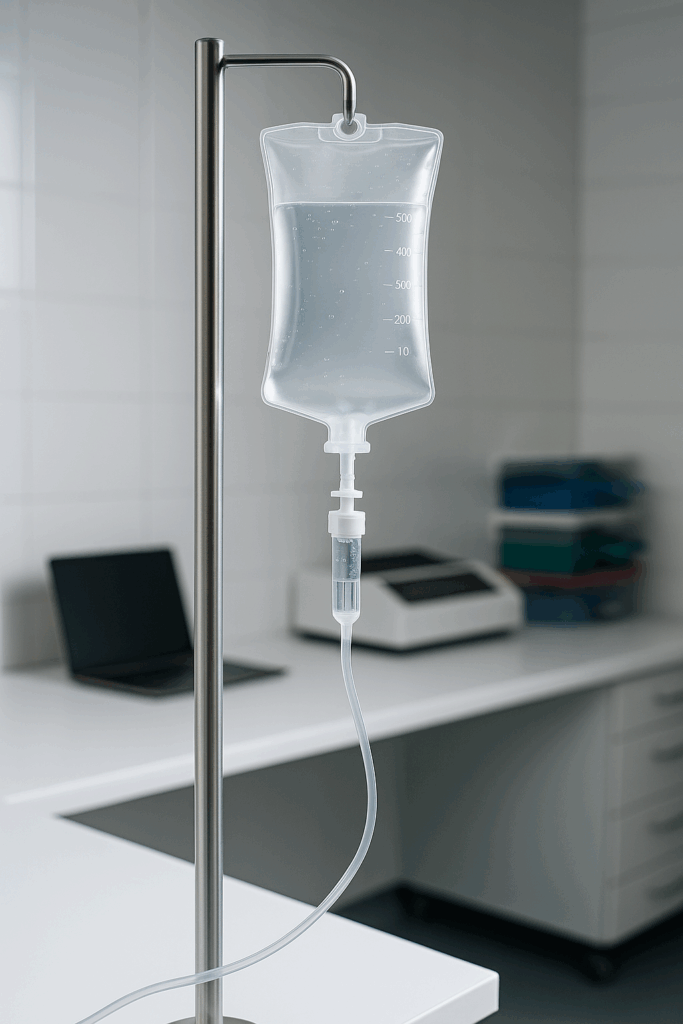
The Raptor 1788 will enable direct sample analysis from IV bags, eliminating the need to transfer samples into beakers or syringes. This non-invasive approach minimizes contamination risk, preserves sample integrity, and allows for long, continuous particle monitoring — something not possible with conventional Light Obscuration systems.
Stay tuned to see how the Raptor 1788 simplifies large-volume analysis while improving data accuracy and workflow efficiency.
The Raptor 1788 will soon make it possible to analyze pre-filled syringes directly, eliminating the need to transfer samples into secondary containers. This approach preserves sample integrity, reduces contamination risk, and enables both particle and silicone droplet detection, identification, classification and concentration within the same test cycle.
By allowing analysis and return of the original sample, this method supports a true non-destructive workflow — ideal for high-value biologics and injectables where every drop counts.
Check back soon to see how Vision Analytical is redefining precision testing for pre-filled syringe applications.